Pharmaceutical Microbiology and its importance
Anup Bajracharya
Microbiology is the scientific study of microorganisms such as bacteria, archaea, algae, fungi, protozoa, viruses, and certain helminths. This field focuses on understanding their structure, functions, classification, and the methods used to either utilize or regulate their behavior. When the principles, methods, and knowledge of microbiology are specifically applied to pharmaceutical processes, it becomes known as Pharmaceutical microbiology.
Pharmaceutical Microbiology is the applied science concerned
with the study of microorganisms that are involved in:
- the
production of pharmaceutical products,
- ensuring
their safety,
- maintaining
their quality and sterility,
- and
preventing microbial contamination during drug development and production.
Pharmaceutical Microbiology can
be defined as the study of microorganisms that are pertinent to the production
of antibiotics, enzymes, vitamins, vaccines, and other pharmaceutical products;
it also incorporates the study of microorganisms that cause pharmaceutical
contaminations, and degradation, deterioration and spoil of pharmaceutical raw
materials and finished products.
Importance
of Pharmaceutical Microbiology
Pharmaceutical Microbiology
is a vital discipline that plays a key
role in ensuring the safety, quality, and effectiveness of pharmaceutical
products. It is essential across every stage of drug development and
production.
1.
Production of Antibiotics, Vaccines, enzymes etc
Microorganisms are used in the
development and production of antibiotics vaccines, enzymes, monoclonal
antibodies, etc. It ensures biosafety and purity of these biological products.
· Production of antibiotics- The most important use is the
production of antibiotics, two third of antibiotics are produced from
microorganisms. The pharmaceutical microbiology concerns with the isolation of
antibiotic producing microorganisms from natural environments such as soil or
water and use them for production of antibiotics through the process of
fermentation. Thus, Microbiology helps in strain selection, fermentation
monitoring, and antibiotic
potency testing.
Production of vaccines- A vaccine is a biological preparation that improves immunity to a particular disease. A vaccine typically contains an agent that resembles a disease-causing microorganism, and is often made from weakened or killed forms of the microbe, its toxins or one of its surface proteins. The agent stimulates the body's immune system to recognize the agent as foreign, destroy it, and "remember" it, so that the immune system can more easily recognize and destroy any of these microorganisms that it later encounters.
Vaccines
are often made from killed or weakened (attenuated) microorganisms, or specific
components like proteins or toxins. Pharmaceutical microbiologists help to
- Select
the right strain of bacteria or virus.
- Grow
them in optimal culture media under controlled conditions (e.g.,
temperature, pH, aeration). Example: Using Salmonella typhi Ty21a strain
for oral typhoid vaccine production.
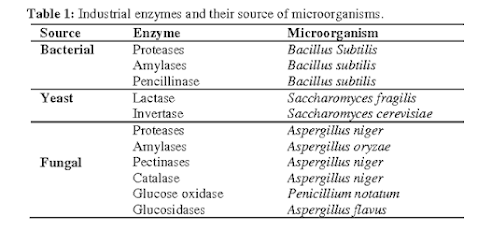
· Production of Enzymes-Microbial cells also produce intracellular
and extracellular enzymes like amylase, proteases, lipases, invertase etc. Enzymes
are collected either from the culture
medium (extracellular enzymes) or from microbial cells (intracellular enzymes).
Examples- Amylase, protease, and lipase:
Included in digestive formulations for patients with poor digestion. Streptokinase and urokinase helps to dssolve blood clots
(used in heart attack treatment).Glucose
oxidase is used in glucose biosensors for diabetics. DNA polymerases from Thermus
aquaticus (Taq polymerase) is essential in PCR (Polymerase Chain Reaction) for genetic testing and disease
diagnosis.
· Production of Alcoholic products- Many microbial cells convert raw
materials or substrates into valuable organic compounds such as butanol, ethanol,
acetone etc. The production of alcoholic beverages and products is achieved
through fermentation, a
metabolic process in which microorganisms—mainly yeasts and bacteria—convert sugars into alcohol (ethanol) and other byproducts like carbon
dioxide.
· Production of Probiotics – Probiotics are live bacteria that may
confer a health benefit on the host. Fuller in 1989 described probiotics as
"live microbial feed supplement which beneficially affects the host animal
by improving its intestinal microbial balance
Lactic acid bacteria (LAB) and Bifidobacteria are the most common types of
microbes used as probiotics, but certain yeasts and bacilli may also be used.
Probiotics are commonly consumed as part of fermented foods with specially
added active live cultures, such as in yogurt, soy yogurt, or as dietary
supplements.
2. Sterility Testing
Sterility testing is a critical
quality control process used to ensure that sterile pharmaceutical products
such as injectables, ophthalmic solutions, and surgical implants are completely
free from any viable microorganisms. This test is especially important for
products that are introduced directly into sterile areas of the body, like blood,
eyes, or tissues, where even a single contaminating microbe can cause severe
infection or sepsis. The process involves incubating samples of the product in
specially prepared culture media, such as Fluid Thioglycollate Medium (FTM) and
Soybean-Casein Digest Medium (SCDM), under controlled conditions for at least
14 days to observe microbial growth. For instance, an intravenous infusion must
undergo sterility testing to confirm that it does not contain any bacteria or
fungi before it can be released to the market.
3. Microbial Contamination Control
Microbial
contamination control is essential for non-sterile pharmaceutical products like
tablets, capsules, syrups, creams, and ointments, which may be exposed to the
environment during manufacturing and packaging. This process involves regular
environmental monitoring, personnel hygiene checks, and testing of raw
materials, in-process materials, and finished products. The goal is to prevent
the presence of objectionable microorganisms, such as Escherichia coli, Pseudomonas
aeruginosa, and Salmonella species, which can cause infections or degrade the
product. For example, Pseudomonas aeruginosa in a topical ointment could infect
wounds and delay healing, hence its presence is unacceptable in such
formulations. Microbial contamination control ensures that the microbial
content stays within acceptable limits and does not pose health risks to
patients.
4.
Microbial Limit Testing
Microbial
Limit Testing (MLT) is used to determine the total number of viable aerobic microorganisms—both bacteria and fungi—present in a
non-sterile pharmaceutical product. This test helps assess whether the
microbial count falls within acceptable limits defined by pharmacopeias (like
USP, BP, or IP). MLT consists of two components: Total Aerobic Microbial Count (TAMC) and Total Yeast and Mold Count (TYMC). Additionally, it includes
specific tests to detect pathogenic
organisms, such as E. coli, Salmonella, and Staphylococcus
aureus. For example, a cough syrup might be tested for its total microbial
load to ensure it contains fewer than 100 colony-forming units (CFU) per mL of
bacteria and fewer than 10 CFU/mL of fungi, ensuring it is safe for oral
consumption. MLT is crucial for maintaining the microbial quality of non-sterile products.
5.
Preservative Effectiveness Testing (PET)
Preservative
Effectiveness Testing, also known as antimicrobial preservative effectiveness
testing, is performed to verify that the preservatives added to multi-use pharmaceutical products are
effective enough to prevent microbial growth during storage and usage. This is
especially important in products like eye
drops, nasal sprays, creams, and syrups, which may be repeatedly exposed
to air or come into contact with users. In this test, the product is
intentionally inoculated with known microorganisms (e.g., Staphylococcus
aureus, Candida albicans, Aspergillus brasiliensis) and
observed over time to see whether the preservatives can eliminate or significantly
reduce microbial growth. For instance, in a multi-dose eye drop, PET ensures
that if bacteria are accidentally introduced during usage, they will not
multiply and compromise the product's safety.
6.
Plays a Role in Innovation
Pharmaceutical
microbiology helps in the discovery of new antimicrobial agents and in
developing rapid diagnostic techniques.
It
is important for addressing antibiotic resistance and ensuring the continued
effectiveness of medicines.
The exploitation of
microorganisms and their products has played an increasingly prominent role in
the diagnosis, treatment and prevention of human diseases. The nonmedical uses
are also of significance, Example, the use of bacterial spores (Bacillus
thuringiensis) and viruses (baculoviruses) to control insect pests, the
fungus Sclerotinia sclerotiorum to kill some common weeds, and
improved varieties of Trichoderma harzianum to protect crops against
fungal infections.
· Diagnosis of diseases and treatment- Different tests are used to
detect infectious microorganisms like ELISA, Widal test. Antimicrobial
Susceptibility testing is mainly used for selection of antibiotics for the
treatment of microbial infections.
· Apart from drugs and bio products development, microbiology contributes towards quality control of a pharmaceutical laboratory. Regular environmental monitoring in manufacturing areas ensures early detection of contamination sources. Personnel hygiene monitoring ensures that staff do not introduce harmful microbes into clean areas.
References
- Denyer,
S. P., Hodges, N. A., & Gorman, S. P. (2004). Hugo and Russell's Pharmaceutical
Microbiology (7th ed.). Blackwell Publishing.
- WHO. (2002). Guidelines
on Good Manufacturing Practices for Pharmaceutical Products. World
Health Organization.




No comments:
Post a Comment